| Diseases of the Esophagus |
The esophagus, a part of the gastrointestinal (GI) tract, is a tubular organ that allows food from the mouth and throat (pharynx) to pass through to the stomach. The esophagus in adults is about 25-30 cm in length. In the neck, the mesopharynx, as it descends, is separated to the anterior structure called larynx which includes vocal cord and is connected to the trachea, and to the posterior structure called hypopharynx which is connected to the esophagus. The esophagus runs approximately along the median line on the posterior side of the trachea from the neck into the thorax. The median structure of the thorax which separates the right and the left lung is called mediastinum. The mediastinum contains the trachea, heart, aorta, and the esophagus. The esophagus is located in the posterior portion of the mediastinum.
In the upper mediastinum, the esophagus is located between the trachea and the vertebra, and is surrounded together with the trachea by the aortic arch. In the middle and lower mediastinum, the esophagus is surrounded by the vertebra on the posterior side, by the heart on the anterior side, by the descending aorta on the left posterior side. It is completely covered with bilateral lung all along the thorax. The esophagus penetrates the diaphragm through the hole called esophageal hiatus and runs into the abdomen. Then the esophagus is connected to the orifice of the stomach (cardia). This complicated anatomical location with many vital organs around makes the surgical approach to the esophagus difficult. (Fig. 1).
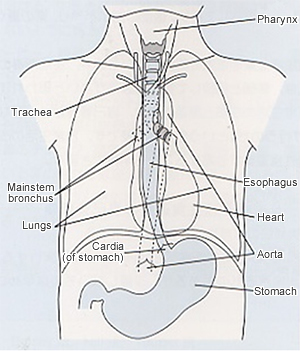
Figure 1: Relationship between the esophagus and surrounding organs
Source: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition.
The esophageal wall consists of several layerd structures, i.e., begining from the luminal side, the epithelium, lamina propria, muscularis mucosa, submucosal layer, proper muscle layer, and the adventitia (Fig. 2). Cancer begins with malignant changes of the epithelial cells.
The esophagus, unlike the rest of the GI tract, plays no role in the digestion of food. The only role of the esophagus is to carry food from the pharynx to the stomach. Esophageal peristalsis by muscle contraction propels the food, with some aid by gravity.
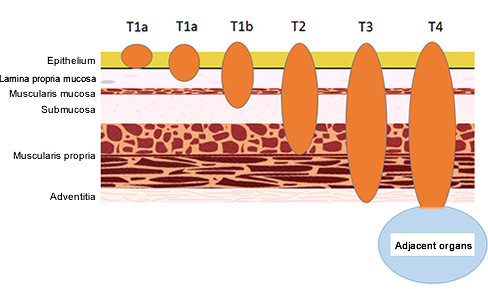
Figure 2: Classification of esophageal cancer by depth of invasion
2.Esophageal cancer
| 1) Summary |
Esophageal cancer (EC) is a typical malignant tumor of the esophagus. More than 90% of ECs in Japan arise in the squamous epithelium and are squamous cell carcinomas (ESCC) that most often develop from the middle to the lower thoracic esophagus. In Western countries, the incidence of ESCC has decreased over the last 30 years, and esophageal adenocarcinoma (EAD) that originates from Barrett's epithelium, now accounts for most ECs. The incidence of EAD may also increase in the future in Japan, but epidemiologic studies have still shown no clear increase in EAD.
A total of 11,592 deaths (9,724 men, 1,868 women) due to EC were reported in Japan in 2012. This represents a death rate due to EC of 9.4 per 100,000 population (15.9 in men, 2.9 in women).1 EC accounts for 3.2% of deaths from all malignant neoplasms. It is the 9th leading cause of cancer death overall, and the 6th leading cause among men. The yearly mortality rate from EC has remained about the same in women, but has increased slightly in men.
| 2) Epidemiology and etiology |
EC occurs most commonly at age 50s to 70s and is more frequent in men, with a male-female ratio of 6:1. Tobacco and alcohol consumption are thought to be the main causes of ESCC. It was recently shown that genetic polymorphisms in alcohol metabolism have been strongly linked to carcinogenesis, in particular, those involving the gene for the enzyme aldehyde dehydrogenase-2 (ALDH2). Variant type of ALDH2 gene is deficient in the enzyme activity. Heterozygosity for the ALDH2 gene (a pair of a wild type and a variant type of ALDH2 gene) is said to increase the risk for EC. ALDH2 degrades acetaldehyde produced by metabolism of alcohol. Complete absence of ALDH2 activity is associated with alcohol intolerance, whereas an active pair of the genes is associated with good alcohol tolerance. Persons who are heterozygous for ALDH2 initially show flushing on their faces, but over time with practice, can gradually tolerate drinking alcohol. Therefore, heavy drinkers in whom the effects of alcohol are more readily apparent are at a higher risk of developing EC.
| 3) Symptoms |
Patients with early EC are often asymptomatic, but some may have pain, discomfort, and heartburn when swallowing. Symptoms when EC progresses include a sticking sensation and inability to swallow food.
| 4) Diagnosis |
Endoscopy is necessary to detect early asymptomatic EC. Remarkable advances have recently been made in endoscopy. Not only can lesions be seen more clearly than with the naked eye, but endoscopy using special light sources and magnification of lesions is now available. For example, narrow band imaging (NBI) endoscopy using a filter can visualize the esophageal mucosal surface and blood vessels in greater detail. Therefore, EC that may be difficult to detect by conventional endoscopy can be recognized as relatively distinct brown areas on NBI endoscopy (Fig. 3).
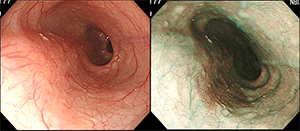
Figure 3: NBI endoscopy
A diagnosis of EC can be confirmed by an endoscopic biopsy. When a lesion is seen on endoscopy, a biopsy sample can be taken and examined for cancer by microscopy. Further evaluation of disease progression, including the spread of cancer and metastases, includes the use of esophagography, X-ray computed tomography (CT) scans, abdominal ultrasound, endoscopic ultrasound, and positron emission tomography-computed tomography (PET-CT). These tests are used to evaluate lesion size, infiltration to other organs, lymph node (LN) metastases, and distant metastases to such as the liver and lung.
The progression (stage) of EC is determined based on extent of cancer invasion into the esophageal wall (depth of wall invasion: T) (Fig. 2), site of LN metastases (N), and distant metastases to other organs (M) (Table 1, Fig. 4). The stage classification shown here is according to the 10th edition of Japanese Classification of Esophageal Cancer.
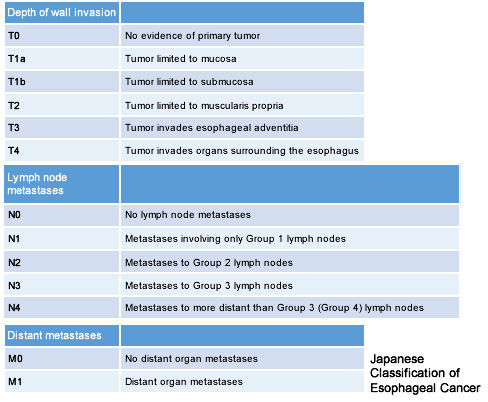
Table 1: Evaluation of esophageal cancer progression
Source: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition.
Refer to the following paper for Lymph Node Groups.
The Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus 2009;6:1–25
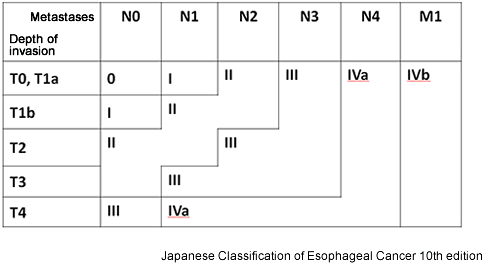
Figure 4: Esophageal cancer progression
Source: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition.
| 5) Treatment |
Early EC (stage 0) is usually treated endoscopically, but more advanced EC is treated with a combination of surgery, radiotherapy, and chemotherapy using anti-cancer drugs. Recommended treatment guidelines based on disease stage are shown below (Fig. 5).
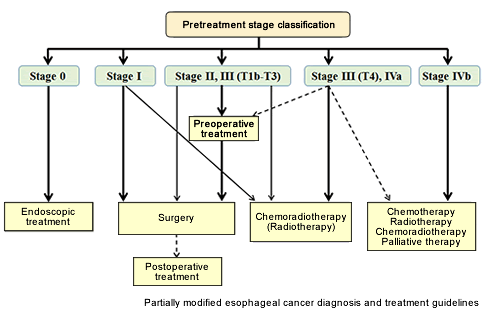
Figure 5: Algorithm for treatment of esophageal cancer
Source: Guidelines for Diagnosis and Treatment of Carcinoma of The Esophagus (in Japanese). April 2012 edition.
- Endoscopic treatment: Endoscopic treatment for EC is indicated when the depth of a tumor arising in the epithelium is limited to the epithelium (in situ) or to the lamina propria. These early ECs almost never have lymph node (LN) metastases. Complete resection of the EC by endoscopy often provides a cure.
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are two types of endoscopic treatment. There are several different type of EMR techniques. The cap-assisted technique, which is one of such EMR techniques, is shown here. The cap-assisted EMR is performed using an endoscope with a transparent cap with claw attached to the tip. Liquid is injected under the cancer to elevate the lesion, a metal loop (snare) is placed around the lesion while aspirating it into the cap, and an electric current is passed to resect the lesion. EMR is a simpler procedure, but the size of cancer that can be resected is limited.
ESD is started with circumferential incision of the mucosa around the lesion using a special knife, then the submucosal layer under the tumor is dissected from the esophageal wall using the knife. ESD enables en bloc dissection of larger EC lesions and is associated with lower recurrence rates (Fig. 6, 7).
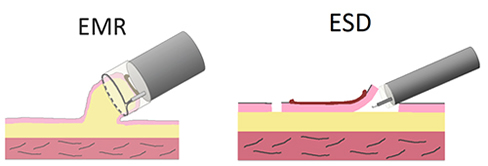
Figure 6: Endoscopic treatment (EMR and ESD)
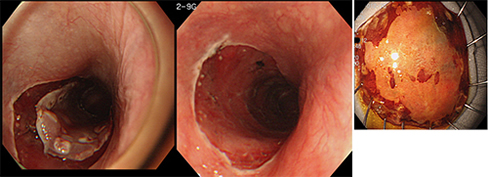
Figure 7: An actual example of ESD
Other endoscopic treatment includes argon laser cautery and YAG laser irradiation, but these are usually performed as additional treatment for residual lesions after EMR or for dilation of strictures. Cautery is also applied in Western countries to Barrett's epithelium for ablation as a prophylactic measure against cancer progression.
- Surgery: EC may be associated with widespread LN metastases at a relatively early stage (Fig. 8). Apparently normal size LNs may widely contain small metastases even when CT and positron emission tomography (PET) scans show no metastases. Therefore, standard (radical) surgery for thoracic EC includes resection of the thoracoabdominal esophagus excluding the cervical esophagus (subtotal esophagectomy) and removal of cervical, thoracic (mediastinal), and abdominal LNs with possible metastases (3-field LN dissection). This is major surgery that also includes reconstruction of the resected esophagus using the stomach, colon, or small intestine (Fig. 9).
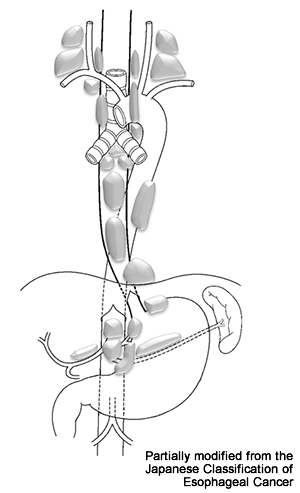
Figure 8: Lymph nodes to which esophageal cancer commonly metastasizes
Source: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition.
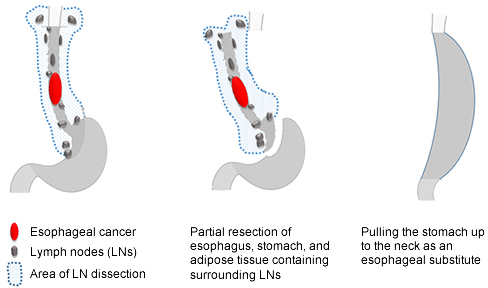
Figure 9: Surgery for esophageal cancer
Source: Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition.
The stomach is most often used for esophageal reconstruction by creating a tube of stomach (creation of gastric tube) and pulling this up to the neck and suturing it with the cervical esophagus. If the stomach is not available, the colon, and more rarely, the small intestine can be used.
One of three routes of esophageal reconstruction is selected: through the mediastinum where the esophagus originally was located (posterior mediastinal route), through a tunnel created behind the sternum (retrosternal route), and through a tunnel created in the anterior thoracic subcutaneous tissue (ante-thoracic route).
Each of these reconstruction routes has advantages and disadvantages. Reconstruction via the retrosternal route is relatively safe and has conventionally been used, but the posterior mediastinal route is becoming more popular because it is more physiological and swallowing food is easier after surgery. Although swallowing may be easier after reconstruction via the posterior mediastinal route, gastric juice reflux is more likely to occur. In addition, suture insufficiency (anastomotic leakage), although not common, can lead to empyema and mediastinitis, which may result in a serious condition.
- Chemoradiotherapy: Chemoradiotherapy, which combines chemotherapy using anti-cancer drugs with radiotherapy, has been shown to be more effective than radiotherapy alone for EC. Chemoradiotherapy is the most promising non-surgical treatment to eradicate EC. Definitive chemoradiotherapy can be indicated even when radical surgery is possible. It is also indicated when surgery seems impractical because of tumor infiltration to adjacent structures such as the trachea and aorta. Treatment outcomes are expected to be equivalent to surgery in patients with relatively early (stage I) EC, but in more advanced EC (stage II and III), treatment outcomes with neoadjuvant (preoperative) chemotherapy + surgery are presumed to be superior to those with chemoradiotherapy.
Early adverse reactions associated with this treatment include nausea, vomiting, myelosuppression, esophagitis, stomatitis, diarrhea, constipation, and radiation pneumonitis. Late adverse reactions include radiation pericarditis, radiation pleuritis, and pleural and pericardial effusions. Surgery (salvage surgery) may be necessary for residual cancer or a regrowth after chemoradiotherapy, but high perioperative mortality rates of about 8% have been reported.
- Chemotherapy: Chemotherapy involves treatment with anti-cancer drugs. Chemotherapy is mainly applied to patients with stage IVb EC in whom surgery is no longer indicated because they have widespread systemic metastases. The goal of treatment is to prevent or delay further disease progression. Chemotherapy is also applied as a neoadjuvant (preoperative) treatment to aim tumor regression. Current standard treatment is a combination of two anti-cancer drugs, 5-FU and cisplatin (CDDP) (FP therapy).
For patients with higher risks such as renal dysfunction or high age, cisplatin may be replaced by nedaplatin, or docetaxel (DTX) alone may be used. Other chemotherapy regimens that are gradually being used recently include DTX/CDDP/5-FU (DCF therapy) and weekly paclitaxel (PTX) (once in a week for 3 weeks followed by one week off). Adverse reactions include nausea, vomiting, loss of appetite, stomatitis, diarrhea, alopecia, leukopenia, and renal dysfunction.
- Palliative treatment: Palliative treatment in patients with surgically unresectable EC include esophagostomy, esophageal bypass, and esophageal stent placement. A cervical esophagostomy can be carried out by dissecting and opening the cervical esophagus to the cervical skin surface. This procedure is often done with simultaneous gastrostomy. Percutaneous trans-esophageal gastrotubing (PTEG) is a new procedure which requires a special kit to puncture the cervical esophagus through the neck skin, followed by insertion of a tube from the puncture site into the stomach through the esophagus. Esophageal stent placement involves insertion of a special stent through a stricture due to EC under X-ray fluoroscopy or endoscopic guide to dilate the stricture. Esophageal stent placement has become applied more often than esophageal bypass.
3.References
| References |
The Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus 2009;6:1–25
Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1–30.
| References (Japanese) |
The Japan Esophageal Society. Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. The 10th edition. Kanehara Shuppan, Tokyo, 2007.
The Japan Esophageal Society. Guidelines for Diagnosis and Treatment of Carcinoma of The Esophagus (in Japanese). April 2012 edition. Kanehara Shuppan, Tokyo, 2012.
